
“Drugs that can give you mood swings” – Reports suggest that beta-blockers and calcium antagonists are medications that can lead to hospitalised mood disorders
Recent reports indicate that individuals taking medication known as beta-blockers and calcium antagonists for a period of more than 90 days may be susceptible to mood disorders that require hospital treatment.
These medications – commonly used to control blood pressure – are said to be a possible cause for major depression and / or bipolar disorder, drawn from comparisons with patients controlling their high blood pressure with angiotensin receptor blockers (ARBs) instead.
Continue Reading…

“Is the iPhone 7 going to be the next Samsung Galaxy Note 7?” – Apple investigating claim of an iPhone 7 bursting into flames
Following the mass recall of the Samsung Galaxy Note 7, which turned out to be susceptible to explosions and fires, Apple may well have enjoyed a competitive advantage off the back of one of their main rivals as Samsung’s sales plummeted and costs to fix their mistake run in to the billions.
But it turns out that this may have been short lived, as reports are suggesting that an Apple iPhone 7 caught on fire in Australia recently.
Continue Reading…

“Refund for the explosive Galaxy Note 7” – Following the ban of the Samsung Galaxy Note 7 on U.S. airplanes, Samsung is offering exchange or refund on the ‘explosive’ devices
You may have heard about the ‘explosive’ devices; A.K.A. the Samsung Galaxy Note 7 mobile phones.
Following reports across the globe that the devices are prone to catching fire, with some even reportedly exploding in China, devices have been understandably banned on flights for fears of incidents in the air. In response, the mobile phone manufacturer has since set up booths in airports as part of their ongoing efforts to rectify the situation, and halted production.
Continue Reading…

“Progestogen-only Pill Drug Recalls” – Teva UK Ltd has recalled drugs due to impurities showing in their routine testing
Teva UK Limited are one of the latest pharmaceutical manufacturers that have recalled a batch of their drugs.
The Nacrez 75 mg film-coated tablets have been recalled due to impurities found as part of their routine testing. The recall is said to be a precautionary measure taken by the company to ensure the safety of their customers.
However, as with any medicine recall, the dangers to human health are always something to closely monitor.
Continue Reading…
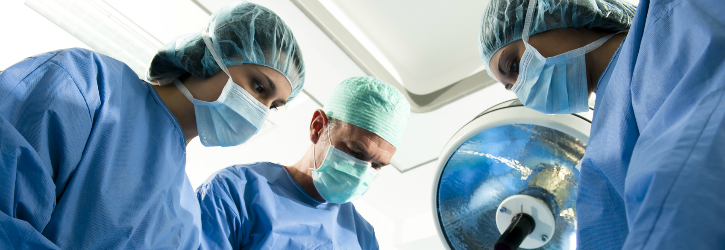
“The Deadly Stockert 3T Machine” – LivaNova machines that have allegedly caused dozens of patients to develop a mycobacterial infection
There are growing concerns surrounding the use of a particular type of heater-cooler system, Stockert 3T, manufactured by LivaNova, which are machines used during a patient’s surgery that have allegedly led to a spate of infections.
This was reportedly the case for Kenneth Piechowski, a Philadelphia man, when he had a surgery to replace a faulty aortic valve back in December 2014.
Continue Reading…

“Good Riddance” – The ban on VW’s diesel vehicle sales in the U.S.
The ban on selling any 2017 diesel vehicles in the U.S., or any diesel vehicles, could be the biggest punishment that VW will have to face from their stunt of cheating emissions tests, which was revealed in September last year.
This could even supersede the millions of dollars in fines that they have to pay out.
The reason why I’m of that opinion is because the diesel vehicles sold in the U.S. made up a big percentage of all VW sales; which is not surprising as the German manufacturer marketed the cars to be “eco-friendly” and “greener” than other vehicles, and they cornered the diesel market well.
However, their “green assertions” turned out to be somewhat misleading…
Continue Reading…

Tracheoesophageal voice devices are thought to only last three months
Tracheoesophageal voice devices have been reported to have a smaller life span than first anticipated.
What was thought to be at least a six month life span recently turned out to be only three months. This is a cause for concern as it could cause greater healthcare costs and a burden for the users who need to keep replacing them.
Continue Reading…

Thousands of recalls for drugs manufactured by Sun Pharmaceutical plants in India and Hungary
Regulators have recently raised concerns surrounding Sun Pharmaceutical’s plants in India and Hungary.
12,000 bottles of beta blocker have been recalled voluntarily by the company when some of the products failed impurities and degradation testing. The mass recall may give rise to legal proceedings if any customer who has bought a potentially defect product from them has had any issues.
Continue Reading…

Samsung Galaxy Note 7 causes evacuation at Louisville Airport due to battery safety concerns
As you’re probably aware, Samsung are having a real nightmare in terms of their Galaxy Note 7 and its tenancy to suddenly set fire to itself…
In one recent incident off the back of this ongoing issue, a supposedly safe device starting ‘smoking’ at Louisville Airport when the owner tried to power off the phone on take-off at an airport. This caused a big disruption to the outbound flight and led to an evacuation.
Of course, the bigger concern is that the faulty product could have caused a serious incident had this have happened during the flight!
Continue Reading…

Mazda recalling up to 42,000 vehicles that may have an airbag dysfunction
There always seems to be a recall in the automotive industry!
One of the latest is yet another problem with airbags for Mazda vehicles, as Mazda North American Operations announces an airbag recall for its Mazda 6 model produced from 4th February 2008 to 3rd December 2009.
Continue Reading…

“Does Bosch Have Something to Hide?” – Volkswagen and Bosch reject access to records in “Dieselgate” scandal
In the latest developments of the Volkswagen Emissions Scandal, and the increasing press surrounding Bosch and their alleged involvement, VW and Bosch have rejected access to more than 20 million pages of records requested by European investors and vehicle owners as part of legal actions.
These documents were submitted to the U.S. courts, and their use for others in Europe could be substantial, which is why they have been requested. However, both German companies have requested that the U.S. federal judge reject any requests made by European investors or vehicle owners asking for the documents.
Continue Reading…

“Recall after Recall” – Hyundai Motor America recalls just over 3,000 2015 Genesis Sedans for Instrument Cluster Defect
Last month, Hyundai was among car manufacturers recalling vehicles, which was due to an instrument cluster defect in its 3,031 2015 Genesis Sedan models. This was picked up by the National Highway Traffic Safety Administration who pressed the automaker to recall this model as soon as possible.
Vehicle recalls are very common. Usually they’re through incidental things which are discovered post-sale, but, on occasions, we see scandals like the VW emissions scandal, where we are acting for thousands of people who have essentially been the victim of a huge bout of misrepresentation.
Continue Reading…