Tag: recalls

Fiat Chrysler Jeep explosion sees campaigners ask for fuel tank recall to be re-investigated
In August 2017, a woman was killed when her Jeep SUV’s fuel tank combusted after she was rear-ended. The incident has raised further concerns that the 2013 recall over the Jeep’s fuel tank was not enough to protect motorists.
The victim’s 2007 Jeep was one of the 1.56 million sport utility vehicles that Fiat Chrysler agreed to install trailer hitches on to protect the fuel tanks. More than five years ago, Chrysler Group recalled 2.7 million sport-utility cars after the vulnerability was linked to the deaths of 51 people after crashes ignited fuel.
Continue Reading…
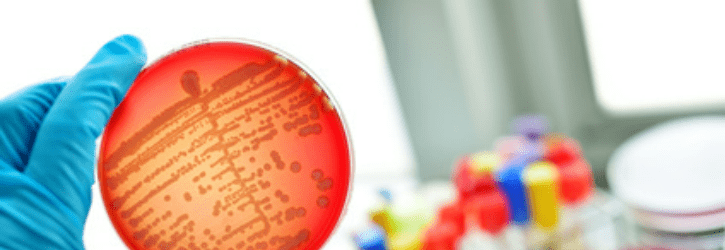
PharmaTech LLC packs up manufacturing plant after its liquid drugs are found to be contaminated with B.Cepacia bacteria
All of PharmaTech LLC’s liquid drugs made at one manufacturing plant have been recalled, with regulators advising consumers not to use them.
Reportedly, the drugs have been contaminated with Burkholderia cepacia bacteria.
Patient infections have been linked to the drugs, meaning regulators are warning against the use of their products. Notably, this is not PharmaTech’s first brush with the law. Regulators have been involved with them three times in less than a year for similar issues involving the bacteria.
Continue Reading…
Medtronic recalls disposable diabetes infusion sets over risk of causing hypoglycemia
Medical device manufacturer Medtronic Plc is recalling disposable diabetes infusion sets as a possible defect may trigger excessive doses of insulin to the diabetic user, putting them at risk of hypoglycemia.
The recall reportedly affects insulin infusion sets distributed all over the world.
The device, connected to an insulin pump, features thin plastic tubing with a needle or cannula at the end to carry insulin into the body when needed. However, apparently, “one in every two million” sets may contain a “complication”.
Continue Reading…

Demands for fidget spinners to be recalled over allegations of toxic lead contamination
Major retailer, Target Corporation, has recalled two of their popular fidget-spinners over reports that they contain excessive levels of toxic lead.
The heavy metal is poisonous and can cause symptoms through exposure like stomach pains, headaches, constipation, irritability, memory problems, and tingling in the hands and feet
The brain is particularly sensitive to lead exposure, and in serious cases, exposure can cause seizures, coma and even death. Survivors of lead poisoning may become anaemic and infertile
Continue Reading…
Rivaroxaban recalled over rogue blister strips of reduced strength tablets
The Medicines and Healthcare Products Regulatory Authority (MHRA) has announced a recall over Xarelto 20mg film-coated tablets made by Strathclyde Pharmaceuticals Ltd. Better known as Rivaroxaban, the tablets are packaged into blister strips with the weight labelled on one side.
A batch is reportedly affected by the recall following reports of rogue blister strips carrying 15mg tablets finding their way into the twin pack 20mg tablets.
The batch number in question is BXHHDR1, due to expire in September 2019. The 28-tablet packets were reportedly first distributed on 18th September 2017.
Continue Reading…

Lidl gravy was once found to have been contaminated with paint thinner ingredient!
Here’s an unusual story from around this time last year. Reportedly, dangerous amounts of Xylene were found in Lidl’s Kania Meat and Kania Chicken Gravy Granules, leading to Lidl recalling the products and offering full refunds to customers.
As the countdown to Christmas has begun, one can only hope we don’t see a repeat of something like that – this year!
We don’t often cover contaminated food stories, but as budget supermarkets are becoming increasingly popular in recent times, we were reminded of this one in last year’s run up to Christmas, and decided to blog it!
Continue Reading…
NovoPen insulin pens recalled over risk of hyperglycaemia
Certain batches of insulin pens manufactured by Novo Nordisk have been recalled as the cartridge holders may not be strong enough to withstand some household cleaners.
The Medicines and Healthcare products Regulatory Agency (MHRA) have issued a medical device alert to notify hospitals and users to return affected batches to Novo Nordisk for replacement.
NovoPen Echo and NovoPen 5 are both affected by the recall. A total of 87 batches in the U.K. have been recalled by way of a field safety notice, citing broken or cracked cartridges as the problem.
Continue Reading…

DePuy Synthes Impactor for PFNA blade recalled over risk of infection
Manufactured by Synthes, the DePuy impactors used for Proximal Femoral Nail Antirotation (PFNA) have been recalled over potentially dangerous risks of infection. PFNA’s are metal devices inserted into the femur bone to strengthen it.
The U.K.’s Medicines and Healthcare Products Regulation Agency (MHRA) has noted a defect with the medical device as a crack in the weld of the handle. The recalled blade is used to insert and position the PFNA in the centre of the bone, and the operating physician uses the handle to grip and position the attached PFNA. It is the handle for the PFNA Blade impactor that can crack, and continued use may mean bodily fluids like blood or bony debris can get inside the device, risking cross-contamination when it’s used for other patients.
Continue Reading…

Amazon’s solar eclipse viewing glasses recalled as potentially counterfeit
“When you look directly at the sun, the intensity of the light and the focus of the light is so great on the retina that it can cook it,” warns President of the American Optometric Association.
Growing up, we’ve always been told not to look directly at the sun. Parents and teachers warn that its burning light could literally blind you. Looking at the sun can cause burns, and blister and crack the cells in your cornea. The consequences of looking directly at a solar eclipse can be far worse, and experts recommend that you must always use appropriate eyewear with special filters to protect your eyes.
But what if the eye protection doesn’t work?
Continue Reading…

Regulators expand investigations into Ford Explorer SUVs over concerns of carbon monoxide leaking
Regulators are investigating Ford Explorer SUVs that may be leaking harmful carbon monoxide from their exhaust systems.
The popular and reputable models are commonly used by U.S police forces, and earlier this year between March and July, six police officers were reportedly taken to hospital to be treated for carbon monoxide poisoning.
Five officers required treatment but were not kept overnight, and the first police officer who was admitted for carbon monoxide poisoning in March was reportedly placed on medical leave for around a month.
Continue Reading…

Britax recalls over 207,000 infant car seats due to a choking risk
Manufacturer of car seats and strollers, Britax, are reportedly recalling a number of their infant car seats as the chest clip between the safety straps may be a choking hazard.
Affected models of infant car seats include the B-Safe 25, BOB B-Safe 35; and the 35 Elite.
It’s thought that the recall is limited to models manufactured between 1st November 2015 and 31st May 2017, and it’s thought to be a pre-emptive one as no injuries have been reported so far.
Continue Reading…

Honda recalls over 24,000 cars after manufacturing defect may cause risk of fuel leak
Honda has issued a voluntary recall for 24,000 cars over concerns that its fuel supply pipes may not be connected properly, or may come apart, and may therefore leak fuel; and pose a risk of fire.
The two models involved in the recall are:
- AWD Honda CR-V Touring
- 2WD Honda CR-V
If the pipes disconnect, flow of fuel could be disrupted, leading to the engine stalling and therefore increasing the risk of an accident. On top of that, as with any fuel leak, there is a risk of fire as well.
Continue Reading…